Drugs and Devices
Ontario Drug Benefit: How Drugs are Considered
- Health Canada's Drug Review Process – for Sale and Marketing of a Drug in Canada
- Non-Cancer Drugs
- Cancer drugs
- Ontario Drug Benefit Program
- Drug Review and Funding Process
Health Canada's Drug Review Process – for Sale and Marketing of a Drug in Canada
Before a drug product is authorized for sale in Canada the drug manufacturer must submit scientific evidence of the product's safety, efficacy and quality to Health Canada for review and approval.
The federal review process can take between one and two years, depending on the nature of the product.
Once Health Canada approves the product for sale in Canada, a Notice of Compliance (NOC) and a Drug Identification Number (DIN) for the product are issued.
More details on Health Canada's process can be found on their website.
Approval of a product for marketing by Health Canada does not automatically mean that it will be publicly funded in Ontario. It is the drug manufacturer's responsibility to seek public drug program funding by filing a complete submission according to the ministry's established evidence-based drug funding review process.
Non-Cancer Drugs
Common Drug Review
To be considered for funding under most public drug plans, a manufacturer must file a submission to the national CDR process. The CDR is a single process for undertaking reviews and providing common listing recommendations for new drugs (except for new cancer drugs), based on rigorous clinical and pharmacoeconomic analyses and patient input, to participating federal, provincial and territorial (F/P/T) drug benefit plans in Canada. All jurisdictions participate except Québec.
The CDR is administered by the Canadian Agency for Drugs and Technologies in Health (CADTH). It receives expert advice from the Canadian Drug Expert Committee (CDEC) which makes recommendations to the participating jurisdictions.
More details on CDR's process can be found on their website.
Cancer drugs
The pan-Canadian Oncology Drug Review
The pan-Canadian Oncology Drug Review (pCODR) is an evidence-based, cancer drug review process.
The pCODR process brings consistency and clarity to the assessment of cancer drugs by reviewing clinical evidence, cost-effectiveness, and patient perspectives, and using this information to make recommendations to Canada's provinces and territories (except Quebec) in guiding their drug funding decisions. pCODR assesses cancer drugs and recommends which ones Ontario and other provinces and territories in Canada (except Quebec) should consider funding under their public drug programs.
pCODR is administered by the Canadian Agency for Drugs and Technologies in Health (CADTH). It receives expert advice from the pCODR Expert Review Committee (pERC), which makes recommendations to the participating jurisdictions regarding coverage.
More details on pCODR’s process can be found on their website.
Ontario Drug Benefit Program
Drugs funded through Ontario Drug Benefit Program are either listed in the Ontario Drug Benefit (ODB) Formulary/Comparative Drug Index (the "Formulary") or are approved on a case-by-case basis through the Exceptional Access Program (EAP).
The ministry provides drug coverage for over 4,400 quality-assured drug products to eligible recipients. While the list of drug benefits in the Formulary is extensive, it does not include every drug, indication, strength and dosage form that a physician may prescribe.
Use search tool to find out what drugs are covered through the ODB program, including OHIP+.
New Drug Funding Program
The New Drug Funding Program (NDFP) is administered by Cancer Care Ontario (CCO) and provides funding for certain injectable cancer drugs that are administered in hospitals and cancer care centers.
Ontario's Drug Review Process – for Funding through the ODB Program and the NDFP
Drugs are considered for funding in Ontario based on a thorough assessment of the scientific, clinical evidence and patient perspective, as well as the impact on health services as compared to existing treatments in Ontario.
New drug products, including requests for different indications of existing drug products, may be considered for funding in Ontario if the manufacturer makes a complete submission to the ministry.
In general, new drugs and new indications that are approved by Health Canada are first reviewed under the national Common Drug Review (CDR) process, with overall assessment of the clinical, pharmacoeconomic and patient evidence by the Canadian Drug Expert Committee (CDEC). CDEC then issues a recommendation to participating provincial and territorial drug plans, recommending whether or not, and according to what criteria and/or conditions the drug should be considered for funding by public drug plans. Following the release of the final CDEC recommendation, it is up to each province to decide whether to fund the drug product as a benefit under its own provincial drug plan(s). The pan-Canadian Oncology Drug Review (pCODR) and the pCODR Expert Review Committee (pERC) perform the same function for cancer drugs.
Effective April 1, 2016, Ontario streamlined the drug evaluation process for drugs already assessed by the national CDR or pCODR processes to align and reduce duplication in the evaluation process. Ontario-specific drug submissions for funding, i.e. drugs not reviewed by CDR or pCODR, continue to be evaluated by the ministry’s drug expert advisory committee, the Committee to Evaluate Drugs, who provides recommendations and advice to the ministry.
Generic drugs:
Generic drugs have the same active ingredient as the brand name products. Since they have the same active ingredient, generic drugs behave in the same way as brand name equivalents, they can be substituted, or become "interchangeable" with the brand name drug. Generic drugs have the same quality and efficacy as brand name drugs. Generic drugs must meet the same scientific norms and standards as brand name drugs. These standards are set by Health Canada.
Since 2005, the Ministry has made numerous changes to harmonize its generic drug review process with that of Health Canada, thereby streamlining and simplifying the Ontario process for designating generic drugs products as benefits and interchangeable with brand products. Less than five percent of submissions for generic drug products require scientific review by the CED for funding and interchangeability recommendation. Submissions for all generic drug products are required to meet the ministry’s submission requirements before a final decision is made for each product for designation of interchangeability on the Formulary.
pCPA negotiations:
Since 2010, provinces and territories have been working together through the pan-Canadian Pharmaceutical Alliance (pCPA) to conduct joint provincial/territorial negotiations for brand name drugs in Canada. The goal of the pCPA is to improve access to effective medicines, ensure consistency across provinces and territories, and achieve greater value for publicly funded drug programs and patients. All brand name drugs coming forward for funding through the national review processes Common Drug Review (CDR) or pan-Canadian Oncology Drug Review (pCODR) are now considered for negotiation through the pCPA.
For further information, please visit the pCPA website.
Final funding decisions:
The Executive Officer of the Ontario Public Drug Programs makes a final decision on whether or not the drug product should be listed in the Ontario Drug Benefit (ODB) formulary, funded through the ODB’s Exceptional Access Program (EAP) on a case-by-case basis, or funded through the New Drug Funding Program for injectable cancer drugs. A number of factors are taken into consideration in making this decision, such as:
- The expert review committee recommendation (CDR, pERC, and/or CED recommendation)
- Patient and societal impact, public interest
- pCPA negotiations and product listing agreements with manufacturers
- Guidance from other advisory bodies, e.g. Patient and Family Advisory Council
- The sustainability of Ontario’s public drug programs
The Minister of Health may review expert advisory committee recommendations made to the Executive Officer as well as decisions made by the Executive Officer not to designate a product on the Formulary following a positive recommendation from an expert advisory committee. The Minister would receive the expert advisory committee’s recommendations and, where applicable, any reasons for the Executive Officer’s decision not to designate a product. The final decision is made by the Executive Officer.
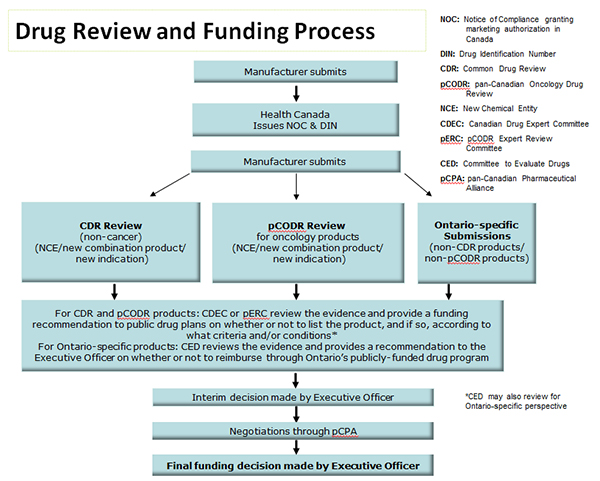
Drug Review and Funding Process
| Manufacturer submits | ||
| Health Canada Issues NOC & DIN | ||
| Manufacturer submits | ||
| CDR Review (non-cancer) (NCE/new combination product/ new indication) |
pCODR Review for oncology products (NCE/new combination product/ new indication) |
Ontario-specific
Submissions (non-CDR products/ non-pCODR products) |
For CDR and pCODR products: CDEC or pERC review the evidence and provide a funding recommendation to public drug plans on whether or not to list the product, and if so, according to what criteria and/or conditions* For Ontario-specific products: CED reviews the evidence and provides a recommendation to the Executive Officer on whether or not to reimburse through Ontario’s publicly-funded drug program |
||
| Interim decision made by Executive Officer | ||
| Negotiations through pCPA | ||
| Final funding decision made by Executive Officer | ||
*CED may also review for Ontario-specific perspective
Flow chart representing the process

 Public Information
Public Information